Best Practices for Social and Behavioral Research Based on Gcp
The development of the GCP - Social and Behavioral Research Best Practices for Clinical Research course was supported by the National Center for Advancing Translational Sciences NCATS National Institutes of Health NIH through the Clinical and Translational Science. The basic CRC course is intended as foundational role-based training for learners needing basic CRC training or organizations needing onboarding training for.

Conceptual Framework Of How Good Clinical Practice Gcp Relates To Download Scientific Diagram
This study evaluated the.
. Effective January 1 2017 all NIH-funded investigators and staff who are involved in applying for conducting overseeing or managing clinical trials should. The goal of this course is to introduce good clinical practice GCP principles to clinical andor community-based research investigations involving human subjects as they specifically apply to social and behavioral research. Good Clinical Practice eCourse.
To assure the quality reliability and integrity of data collected 3. GCP is an international ethical and scientific quality standard for designing conducting recording and reporting clinical trials. Based on NIHs eLearning Course.
GCP - Social and Behavioral Research Best Practices for Clinical Research Good Clinical Practice GCP Basic Course SocialBehavioral Research Course Status Incomplete Nat Started Incomplete Passed 07252016 Post-course evaluation Share Question Subjects Research Select Of the based the type Of for Research both groups. The first project phase established recommendations for training in Good Clinical Practice GCP and was done in conjunction with representatives. Social and Behavioral Research Best Practices MICHR.
GCP basic course for social and behavioral research best practices for clinical research. Sure to pass each quiz. This course is presented in a dynamic nine-module format with narration interactive features and downloadable resources.
Introduction Define the role and context of ICH in. To provide standards and guidelines for the conduct of clinical research 4. Social and Behavioral Research Best Practices.
Good Clinical Practice training. The Best Practices in Social and Behavioral Research Course was developed to provide instruction on good clinical practice for social and behavioral trials. This study evaluated the.
Guidance for informed consent procedures Regular team meetings where all study team members have contact with principle investigator to review study progress is. This study evaluated the new course. Best Practices for Social and Behavioral Research.
The goal of this e-learning course is to enable learners to apply good clinical practice GCP principles to clinical research investigations. SBM is pleased to offer free National Institutes of Health NIH training and certification for good clinical practice in social and behavioral research. The GCP Social and Behavioral Research Best Practices for Clinical Research course introduces GCP principles and discusses how they apply to clinical trials using behavioral interventions and social science research.
Good Clinical Practice Ethics Quality Data. The principles of GCP help assure. Early Career Investigators Investigators Clinical Trialists Study Teams.
Results Although concepts underlying GCP were deemed similar across all clinical trials not all areas were equally applicable and the ways in which GCP would be enacted differ for behavioral trials. We have tried to simplify the presentation of GCP as much as possible to make. Developing a competency-based elearning course in good clinical practice Susan Lynn Murphy 1 Christy Byks-Jazayeri 1 Elizabeth Anderson 1 Angela Lyden 1 Jennifer Miner 1 Jordan Hahn 1 and Brandon Lynn 1.
The Foundations of Best Practices The foundation for social and behavioral research best practices are based on ICHs Good Clinical Practice or GCP. In September 2016 the NIH issued a Policy on Good Clinical Practice GCP Training for NIH Awardees Involved in NIH-funded Clinical Trials. Provide researchers with training that applies GCP principles to social and behavioral research.
The principles of GCP help assure the safety integrity and quality of clinical trials. Best practices for social and behavioral research based on Good Clinical Practice GCP include which item below. The goal of this course is to introduce good clinical practice GCP principles to clinical andor community-based research investigations involving human subjects as they specifically apply to social and behavioral research.
Based on NIHs eLearning Course. Investigators and clinical trial staff who are competent in GCP principles will be. The Society of Behavioral Medicine is pleased to offer free National Institutes of Health NIH training and certification for good clinical practice in social and behavioral research.
This group worked with CTSA representatives to tailor competencies and fundamental GCP principles into best practices for social and behavioral research. The Best Practices in Social and Behavioral Research Course was developed to provide instruction on good clinical practice for social and behavioral trials. To protect the rights safety and welfare of humans participating in research 2.
Best Practices in Social and Behavioral Research. Good Clinical Practice for Social and Behavioral Research eLearning Course. This article discusses the process of defining competencies and development of a best practices training course for investigators and clinical research coordinators who conduct social and behavioral research.
Who developed the GCP - Social and Behavioral Research Best Practices for Clinical Research course. Participants across 4 universities took the course n294 and were sent surveys following course completion and 2 months later. The Best Practices in Social and Behavioral Research Course was developed to provide instruction on good clinical practice for social and behavioral trials.
The 12 principles listed below have been adapted from the 13 principles created by ICH and are a great framework for high- quality research and participant safety. The e-Learning course is comprised of nine video course modules and can be accessed in. We have tried to simplify the presentation of GCP as much as possible to make.
A multisite pilot evaluation of the good clinical practice online training course - Volume 2 Issue 2. Good Clinical Practice GCP is an international ethical and scientific quality standard for designing conducting recording and reporting clinical trials. Best practices for social and behavioral research.
Effective January 1 2017 all NIH-funded investigators and staff who are involved in applying for conducting overseeing or managing clinical trials should be trained in good clinical practice. In 2016 the Best Practices in Social and Behavioral Research Course e-Learning Course for GCP was released to.
Good Clinical Practice Gcp Training Ctrin

Google Cloud Storage Infographic Google Cloud Storage Cloud Computing Services Cloud Storage

Good Clinical Practice Gcp Citi Program

Work Group Recommendations To Evaluate Implementation Of Good Clinical Download Table
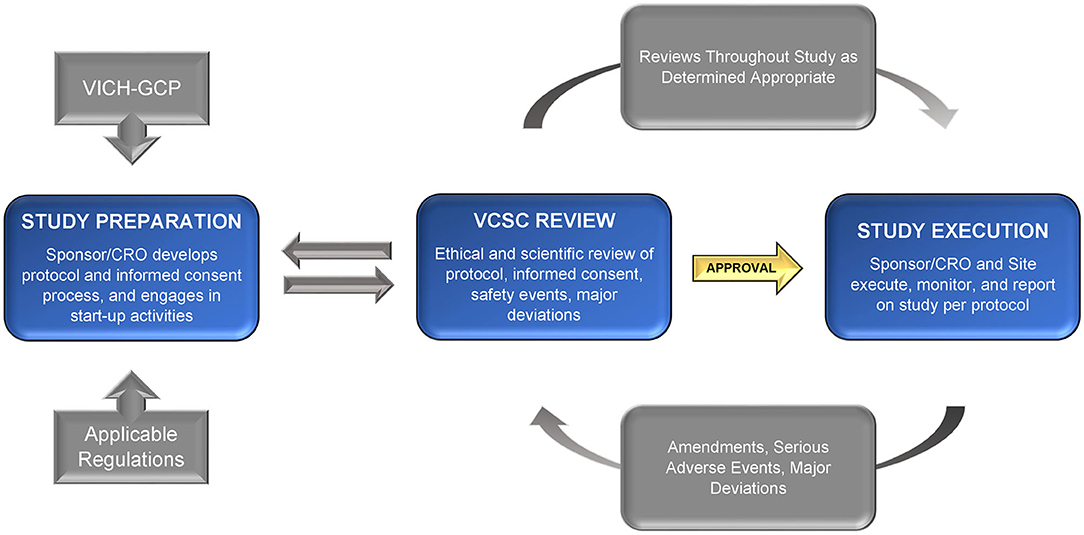
Frontiers Recommendations For Ethical Review Of Veterinary Clinical Trials Veterinary Science

Review Of Gcp Goals Principles Roles And Responsibilities Ich
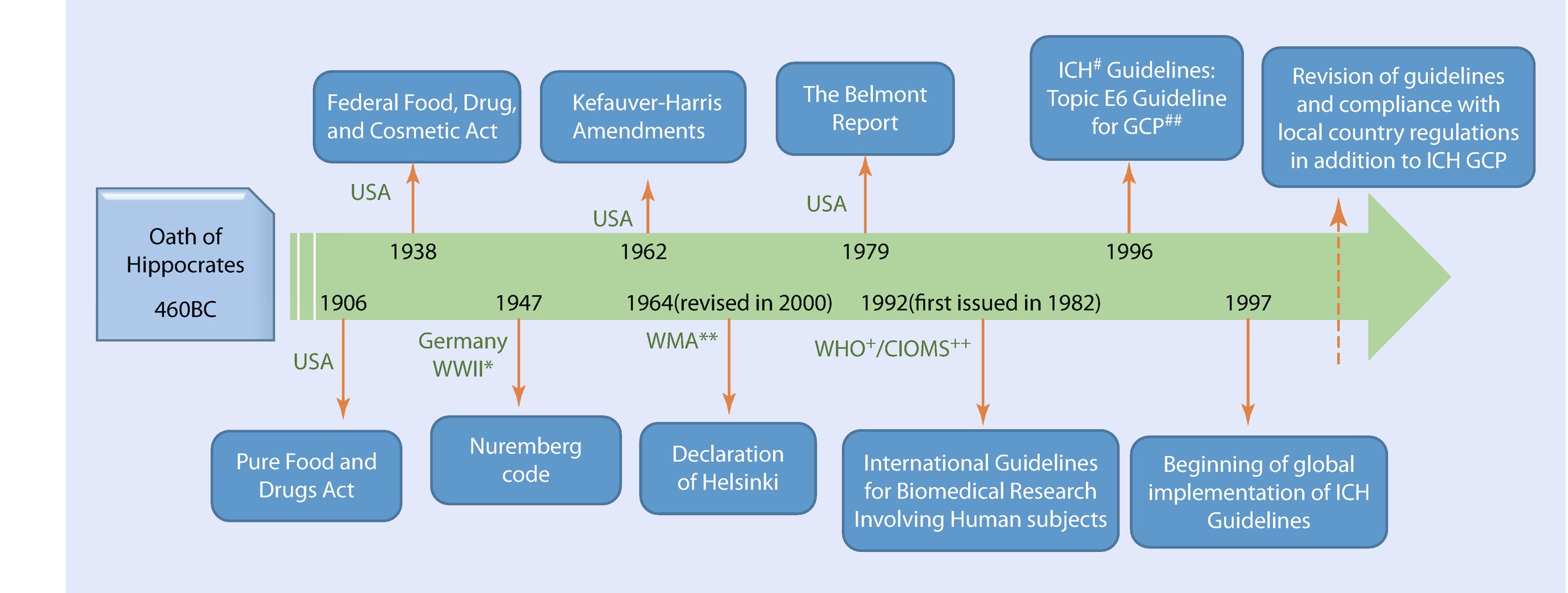
Principles Of Good Clinical Practice Springerlink

Key Principles Of Good Clinical Practice Gcp Part 1 Learngxp Accredited Online Life Science Training Courses

Google Cloud Learning And Certification Path

Bettercloud Is Now Available On Google Cloud Marketplace Gcp

Google Ai Platform In 2022 Reviews Features Pricing Comparison Pat Research B2b Reviews Buying Guides Best Practices

Training Researchers Part 2 Tech Startups Research Studies Ethical Issues

What Is Good Clinical Practice Gcp Training Do I Need It Dementia Researcher

Conceptual Framework Of How Good Clinical Practice Gcp Relates To Download Scientific Diagram

Instructions For Completing Good Clinical Practices Training Office Of Research Support And Compliance

Protocol Training Is Always Required For Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification

Pdf The Need For Good Clinical Practice In Health Care Research
![]()
Gcp Good Clinical Practice Concept With Keywords Letters And Icons Flat Vector Illustration Isolated On White Stock Vector Illustration Of Drug Banner 135041992
Comments
Post a Comment